HPC SYSTEMS Inc. is actively taking on challenges in the field of multiscale simulations, applying computational chemistry [1] technologies to the CAE [2] field, which has become indispensable in various industrial sectors. This work is being carried out to expand the use of computational chemistry. As part of this effort, quantum chemistry calculation [3] has been applied to the chemical reaction model of the rotating disk CVD [4] process, which is commonly used in semiconductor manufacturing and related fields, to refine the model and verify its validity. Through this endeavor, the potential for utilizing precise calculation results obtained from quantum chemistry as input parameters for reaction-flow analysis [5] has been uncovered. HPC SYSTEMS Inc. is actively continuing to explore these PoC [6] projects.
Table of contents
1.Improvement of Chemical Reaction Models for Reaction-Flow Analysis by Quantum Chemistry Calculation
In this study, the chemical reaction model of the rotating disk CVD process [7], a use case of
Chemkin-Pro [8], has been re-evaluated using quantum chemistry calculation to verify its validity for
reaction-flow analysis. Specifically, the calculation has focused on the gas-phase reactions within the
model (Figure 1).
For the evaluation, activation energies [9] for each elementary reaction have been
calculated using the quantum chemistry method, which enables highly accurate energy calculation, and
compared with the activation energy values reported in the previous paper (Table 1). Since these
activation energies serve as key parameters in reaction-flow analysis, the precise energy values obtained
through quantum chemistry calculation are expected to improve the prediction of interactions between
chemical reactions and fluid dynamics with higher accuracy.
Figure 1The case of
forming a silicon thin film on the surface of a rotating disk by supplying a source gas containing
silicon to the central rotating disk [7]
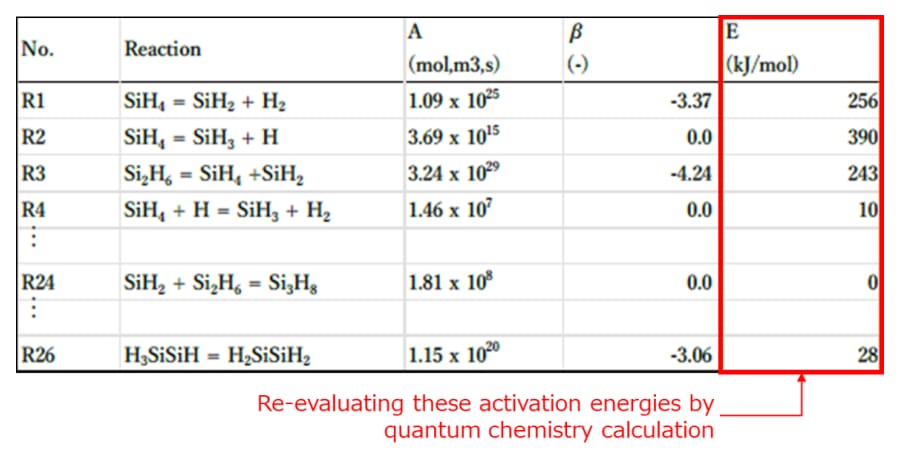
Table 1 Activation
energies for each elementary reaction [10]
2.Investigation Results of Quantum Chemistry Calculation
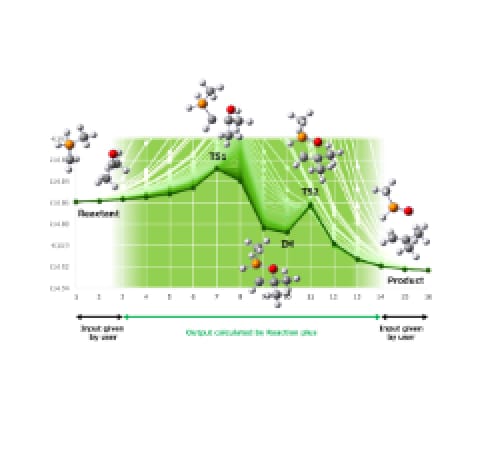
The activation energies have been obtained through the mechanism study of each elementary reaction using quantum chemistry calculation (Figure 2). The comparison with those reported in the previous paper have shown differences, indicating that the potential for utilizing the precise energy values derived from quantum chemistry calculation as input parameters for reaction-flow analysis (Figure 3).
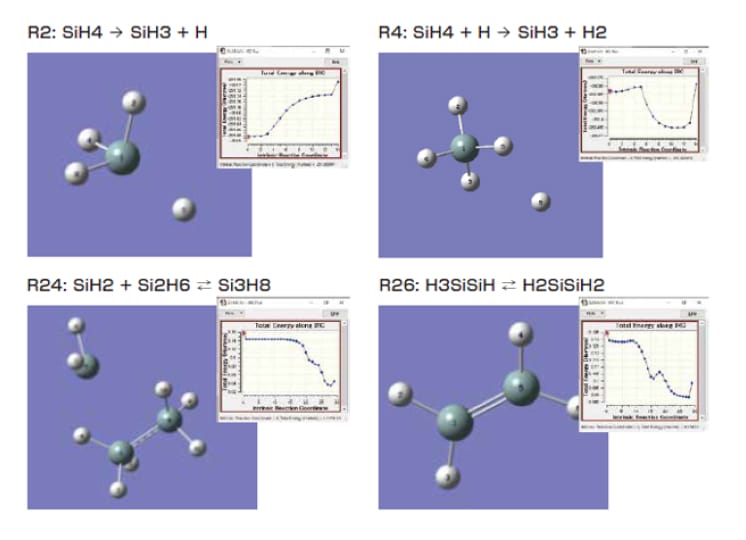
Figure 2Mechanisms
of Elementary Reactions (Partial Results)
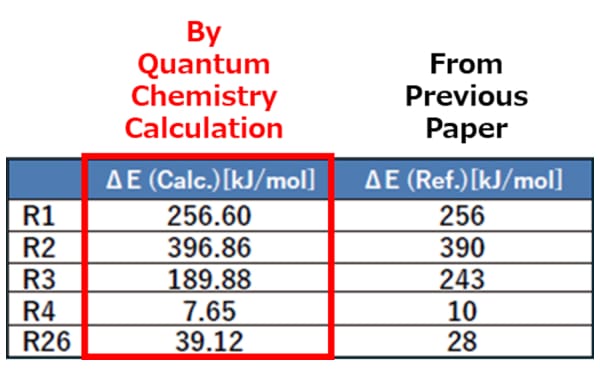
Figure 3Activation
Energies of Elementary Reactions (Partial Results)
3.Keywords & References
[1] Computational Chemistry
The field that uses computer simulation to study atomic and molecular systems as well as chemical reactions. It focuses on understanding and predicting the behavior of atoms and molecules through simulation.
[2] CAE (Computer-Aided Engineering)
The use of software tools to simulate and analyze engineering processes. Primarily used in design and development within the manufacturing industry, CAE has become indispensable across various industrial sectors. Unlike computational chemistry, which focuses on atomic and molecular systems, CAE typically deals with macroscopic engineering applications.
[3] Quantum Chemistry Calculation
The computational method based on the principles of quantum chemistry, which accurately describes the behavior of atoms and molecules. As a part of computational chemistry, it enables precise calculation of molecular properties and reactions.
[4] CVD (Chemical Vapor Deposition)
The process used to create thin films on surfaces through chemical reactions in the gas phase. This technique is widely applied in semiconductor manufacturing and other industrial fields.
[5] Reaction-Flow Analysis
The multiscale analysis method that deals with both chemical reactions and fluid flow, serving to understand their interactions. It integrates macroscopic and microscopic elements to provide a comprehensive understanding of reaction systems.
[6] PoC (Proof of Concept)
The general term referring to a preliminary test or demonstration that verifies the feasibility of an idea, method, or concept before full-scale implementation.
[7] Source Paper
[8] Chemkin-Pro
The software tool provided by Ansys that is widely used for modeling and analyzing chemical kinetics and
reaction mechanisms. It is particularly used for applications such as combustion analysis and other
chemically reacting flow simulations.
Ansys Chemkin-Pro
Website
[9] Activation Evergy
The minimum energy required for a chemical reaction to occur. It plays a critical role in reaction
kinetics, as it determines the rate of a reaction and influences how reaction rates change under different
conditions.
In reaction-flow analysis, activation energy values are used in the Arrhenius equation, the
formula that relates reaction rates to temperature and activation energy. By accurately determining these
values through quantum chemistry calculation, reaction rates can be simulated with high precision. These
simulated rates can then be incorporated into fluid simulation, enabling a detailed understanding of the
interactions between chemical reactions and fluid flow. This integration is particularly valuable in
processes such as combustion, chemical vapor deposition (CVD), and other industrial applications.
[10] Reference Table
Table 2 described in the previous paper [7]
4. For More Info
This article will be updated as needed to include more detailed information. If you are interested in this article, please do not hesitate to contact us through our Contact page.
Last Update
First released on Dec. 20, 2024